As I am jumping into a new project on coral reefs in the Carribeans, I thought it would make great sense for me to spend some time to get some basic ideas about corals, what’s currently threatening them and what are the consequences of the worldwide decline of coral cover.
Some basic knowledge about corals
Corals are cnidarians, Cnidaria is a phylum that includes hydrae (Hydrozoa), jellyfish (Medusozoa), among others. Corals form colonies made of clones called polyps that are linked by a tissue called coenenchyme1. The process of forming a new clone is called budding (or fragmentation). Corals also reproduce sexually and this is how new colonies are created. Just as the other cnidarians, corals have cnidocytes that are cells that produce cnidae (or cnidocysts) which are large and complex organelles2 that allow polyps to catch/kill their prey (zooplankton).
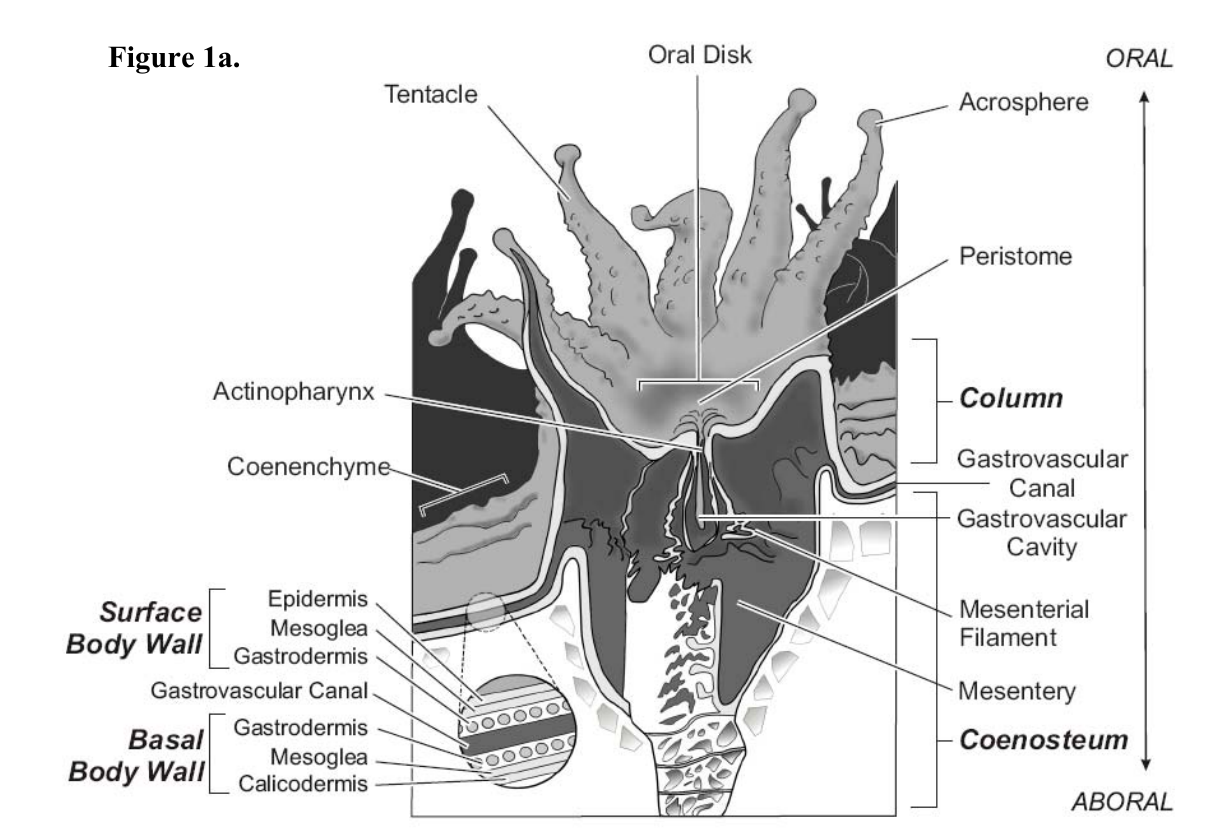
Currently, corals are classified into two main branches3:
Scleractinia, an order that includes hard corals (or stony corals)
Alcyonacea, which includes soft coral and gorgonian corals.
Note that according to the figure below, which summarizes the current the phylogenetic classification of corals, the term “corals” does not represent a monophyletic group4.
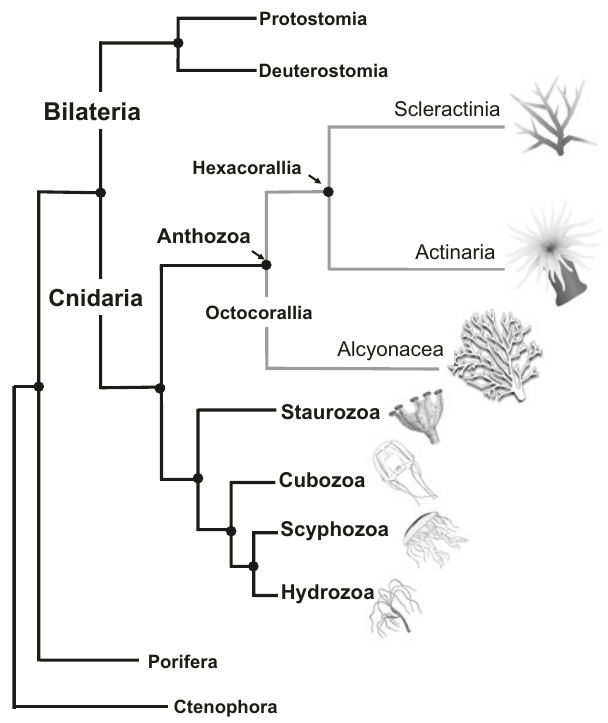
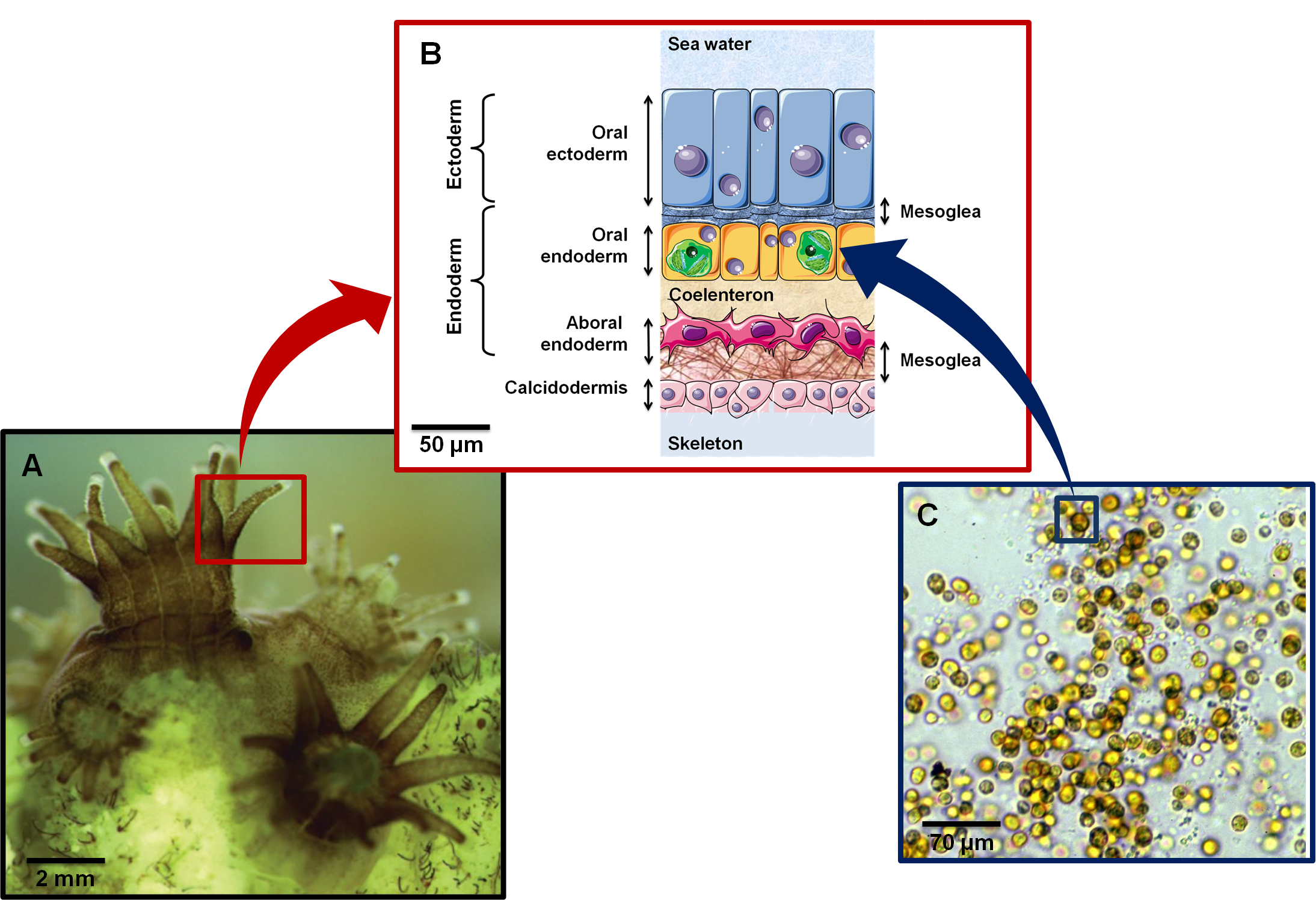
In what follows, I am focusing on hard corals (Scleractinia) that I will sometimes refer to as corals. Corals are reef-building corals, as they grow (via budding), they produce an exoskeleton made of aragonite (CaCO3). Skeletal structures and micro-structures vary strongly among hard corals and have long been the primary determinant of coral taxonomy5. To grow, corals have to feed. As mentioned above, hard corals feed on zooplankton6 and they are also able to adsorb dissolved organic matter. But the main source of carbon is provided by their endosymbionts, zooxanthellae (dinoflagelates) of the genus Symbiodinium (see figure above). Zooxanthellae are single-celled photosythetic organism of ~10 μm. On top of providing carbon as carbohydrates and lipids to their host, zooxanthellae color corals: while coral tissues are rather transparent, zooxanthella chloroplasts have colorful pigments (e.g. peridin) that are associated with chlorophyll and contribute to the photosynthesis. Hence reef-building corals are mixotroph, and it has recently been shown by Fox et al. (2018)7 that corals were capable of adapting their strategy to the environment: in more productive environments, corals feed more on zooplankton.
Corals in the Anthropocene
Over the past few decades corals (and hard corals in particular) have been under several threats and have already severely declined8. The most severe threat to coral reef (and presumably the most famous one) is bleaching. As explained in Rosenberg et al. (2013)9:
Coral bleaching is the disruption of the symbiosis between the coral host and its endosymbiotic algae.
Following the disruption, i.e. the loss of their endosymbionts and the pigments they contain, corals lose their colors, and as the aragonite of their exoskeleton is white, they appear white (which explains the term “bleaching”). Coral bleaching could be seen as a disease, a disease that has many causes. Recent massive and pervasive bleaching events10 have been linked with sustained water temperature rises which cause irreversible damage to the endosymbionts leading to the disruption of the symbiosis. Corals do not necessarily die following the bleaching event, there is a short period of time that allows for a recovery, i.e. for the restoration of the symbiosis. Once passed this period, the colony dies11.
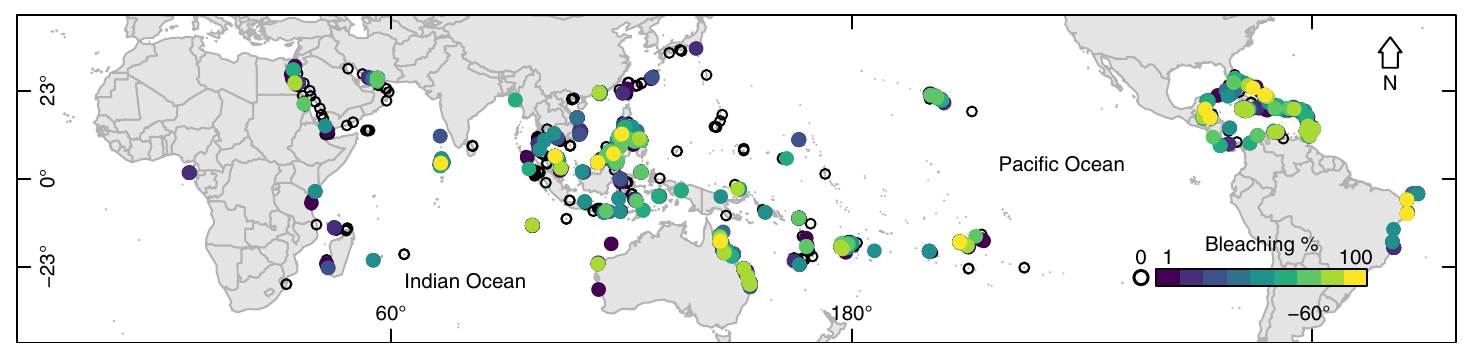
Generally speaking, important variations in environmental conditions (e.g. salinity, concentration of nutrients, etc.) may damage corals and/or the symbiont and may therefore cause bleaching. It has recently been shown experimentally than an imbalanced in the ratio Nitrogen/Phosphorus lowers the thermal tolerance of corals to bleaching12. Hence, anthropogenic eutrophication promotes bleaching events and a recent study in Florida synthesizing 3 decades of observations is very consistent with these findings13. It has also been shown that certain micro-organisms that infect corals can induce coral bleaching9.
Bleaching is a major threat to corals and it has major impact on large and species rich ecosystems. Ecological dynamics are being destabilized and this might lead to even more negative impacts on coral reefs. In the new project I am working on, we are focusing on the ecological interactions in coral reefs and I will expand on this in the next section. The important point here being that coral reefs are in severe danger and as cogently stated by Mumby & Steneck (2008)14:
The paradigm of widespread healthy, stable coral reef ecosystems has evolved to one that views them as patchy, unstable and fragile.
Corals, algal turf, macroalgae and parrot fishes
By building reefs, corals actually create complex habitats that hundreds of fish species inhabit. When corals die, erosion alter the hard skeletons they have left. This can be a slow and steady process, but extreme events, such as storms, can tear off large chunks of aragonite instantly. A major consequence of the death of corals is the loss of structural complexity, which is often quantified by rugosity. As explained in Alvarez-Filip et al. (2009)15
The rugosity index is expressed as the ratio between the total length of a chain and the length of the same chain when moulded to a reef surface.
It has been demonstrated in the Caribbeans that rugosity has drastically decreased since the 70’s. The figure below shows that the decline of rugosity in the Caribbean was actually 3 phases process: 1- loss due to a disease that affect most of the reefs, 2- stable phase (less erosion), 3- decline likely due to bleaching events15.
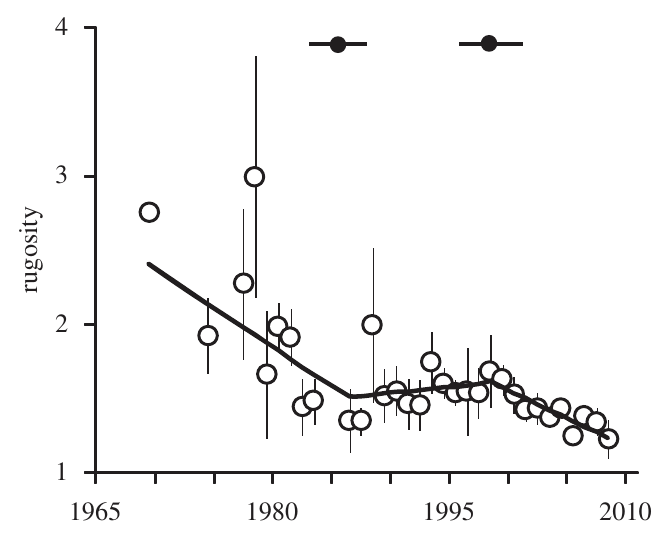
Losing complexity is damaging for biodiversity. Healthy and complex reefs offer a wide array of habitats that suit hundreds of fish species at various (when not all) stages of their life cycle. It has been demonstrated that the higher the structural complexity, the higher the fish biodiversity16 making the loss of structural complexity quite problematic for human populations that depend on fishing.
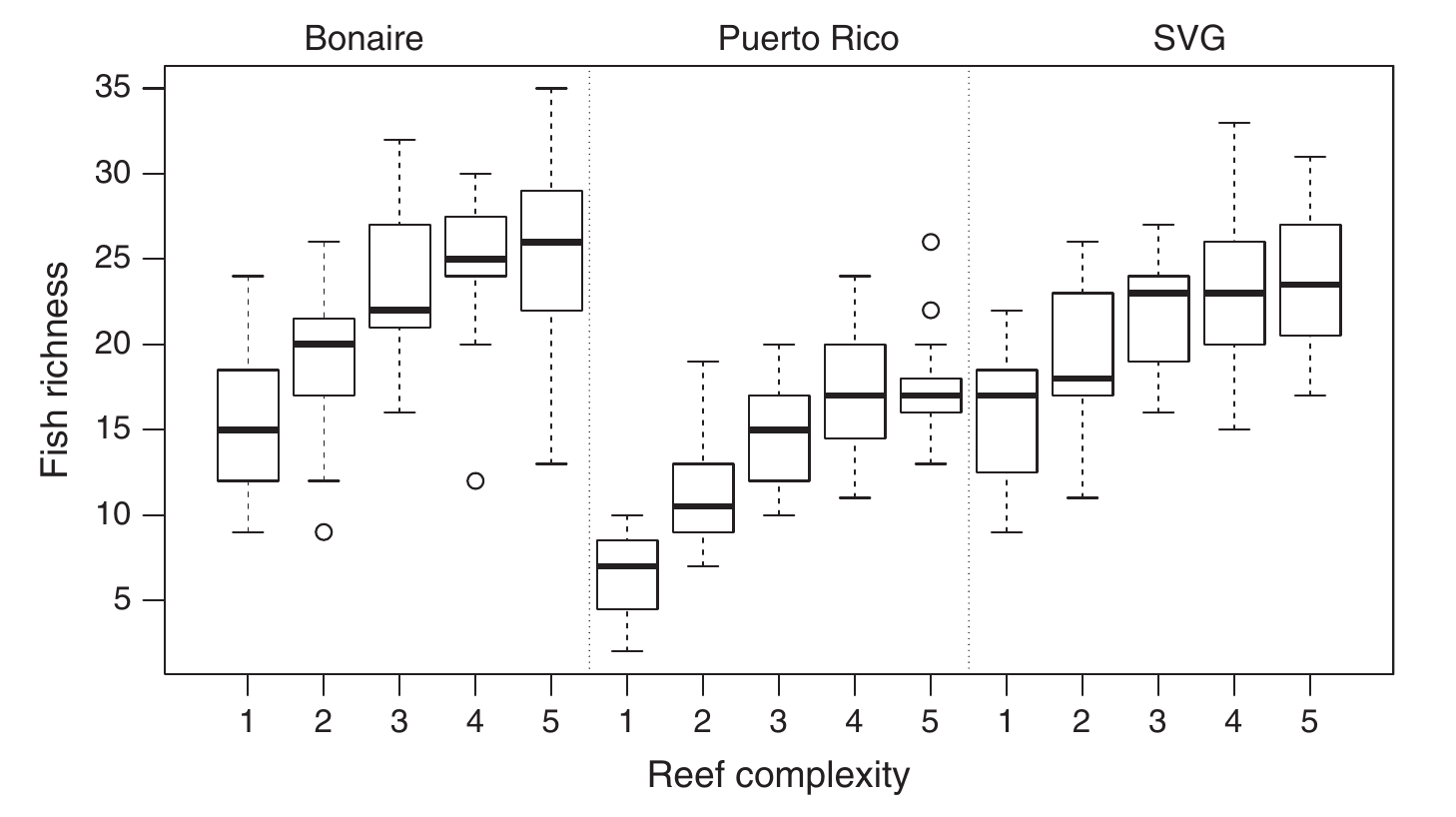
What makes this even more interesting (and even more problematic) is that fish also contribute to the maintenance of coral-reefs, at least in the Caribbean17. To understand how, we need to have a look at the bigger picture. The dynamic of corals-reefs in the Caribbeans have been successfully modeled using 3 types of cover18,19:
- reef-building corals;
- algal turf: a group that includes various short algae, including the crustose coralline alga (CCA) that red algae that play important important structural and biotic roles in the reef20;
- macroalgae: that are important sources of food for several grazers species.
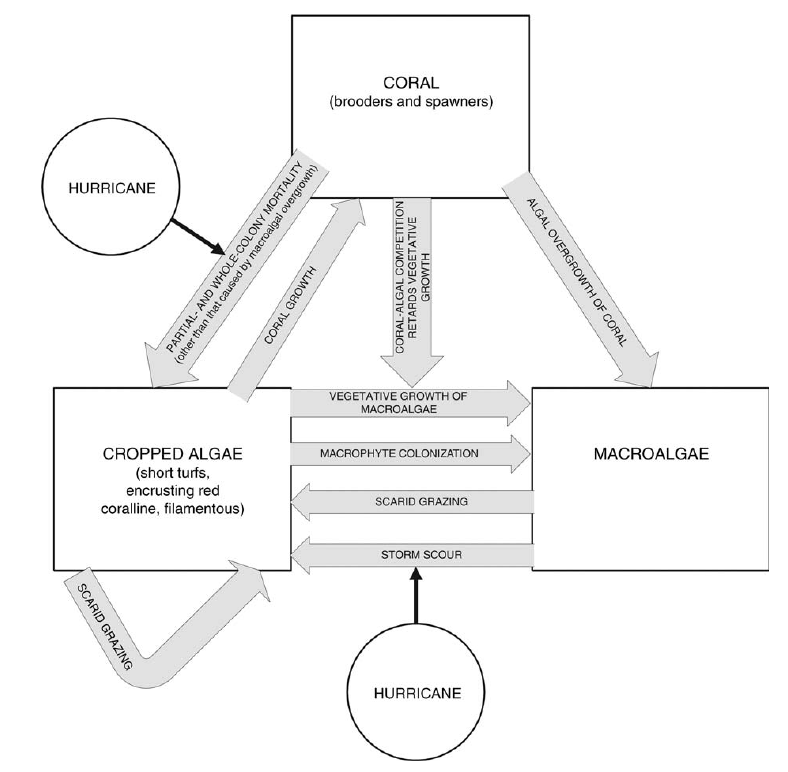
On every reef, there is a dynamical equilibrium between these 3 groups that are competing for space. This equilibrium implies various abiotic and biotic components19 (see figure above). For instance, storms may uproot macroalgae and tear off coral colonies and thereby promote the spread of turf that is less impacted by such events. Parrot fish, as they graze on turfs and macroalgae (this varies among genus, e.g. species of the genus Scarus mainly graze on algal turfs whereas species of the Sparisoma genus have a more varied diet21) promote the growth of corals. This has actually been tested and validated experimentally22 and fossil records support this mechanism23.
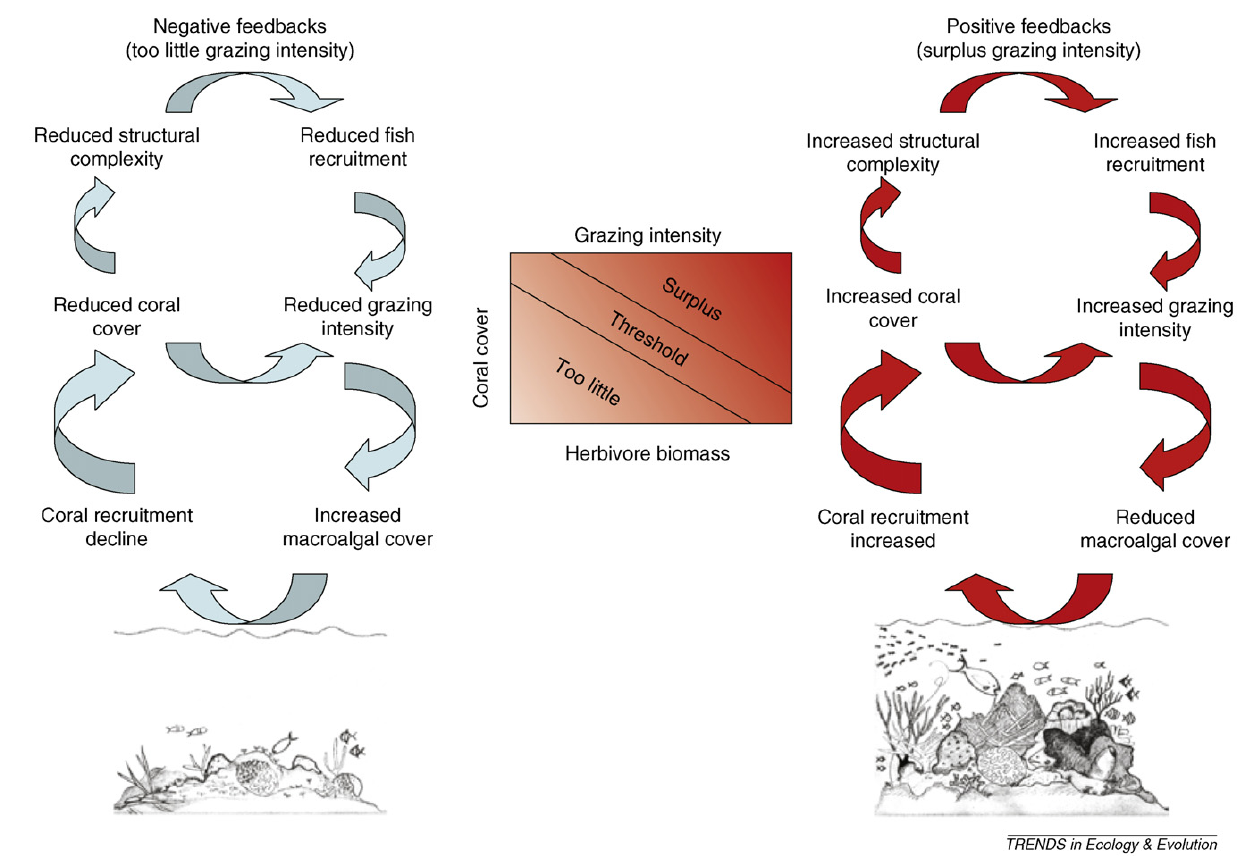
Even more interesting, this dynamic presents a strong hysteresis effect18. In this case, it has been demonstrated that starting from a state where coral cover is relatively high, decreasing the grazing intensity lead to an alternative state where coral cover is low (see figure below). The hysteresis effect is that in order to return to the state where coral cover is high, the grazing intensity has to be significantly greater than what it was when the state changed from higher coral cover to low coral cover. Hence, in the simple situation where fishing pressure was the sole responsible for the change in coral cover, then it would mean that in order to restore the coral cover to what it was previously, the fishing pressure would have to be reduce strongly, presumably to what it was decades ago. Obviously, this is not as simple as reducing fishing pressure, several other factors affect the dynamics, making the problem of protecting corals even harder. For instance, on top of its role in bleaching events, nutrient enrichment can favor overgrowth of algal turfs24.
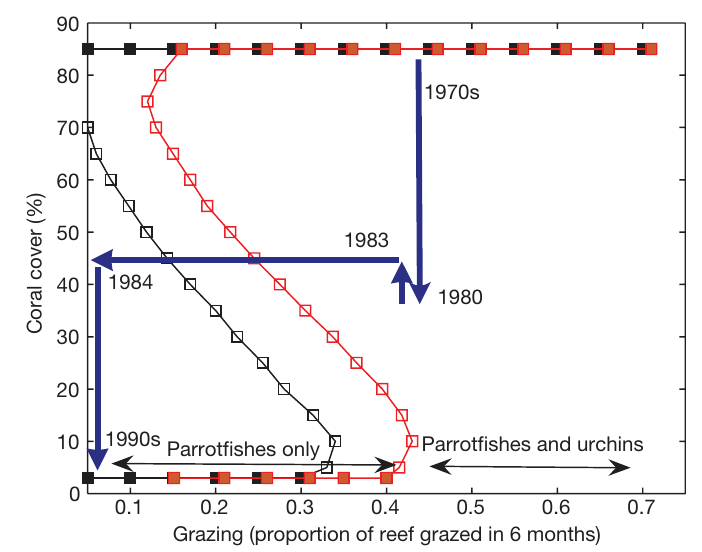
So here I am, working on a complex system impacted by human activities is so many ways. Abiotic, biotic and human factors can play various roles and favor any of the three compartments. The resulting changes in cover will continue to have drastic impacts on the biodiversity in the Caribbeans, which ultimately alters the ecosystem services the reefs provide. I hope to be able to help understanding what are the most important threats and what mechanisms should be primary targets for management actions. Mitigating the impacts of human activities without such information would be inefficient (at least), but efficiency is mandatory to successfully protect what is left of Caribbeans coral reefs.
I have also found the term coeanosarc, e.g. https://fr.wikipedia.org/wiki/Coenosarc. ↩︎
Galloway et al. (2007) Coral Disease and Health Workshop: Coral Histology II https://www.coris.noaa.gov/activities/cdhc_histo_II/rpt_full.pdf ↩︎
Palmer & Traylor-Knowles (2018) 10.1007/978-3-319-76768-0_3 ↩︎
Unless we assume that “corals” also encompass Actinaria (sea anemones). ↩︎
Stolarski & Roniewicz (2001) 10.1666/0022-3360(2001)075<1090:TANSOE>2.0.CO;2 ↩︎
Some species can feed on phytoplankton. ↩︎
Fox et. al. (2018) 10.1016/j.cub.2018.08.057 ↩︎
Dietzel et al. (2020) 10.1098/rspb.2009.0339 ↩︎
Rosenberg et al. (2013) 10.1038/ismej.2008 . ↩︎ ↩︎
Sully et al. (2019) 10.1038/s41467-019-09238-2 ↩︎
I am not entirely sure how it works if only a part of the colony is affected. ↩︎
Wiedenmann et al. (2013) 10.1038/NCLIMATE1661 ↩︎
La Pointe et al. (2019) 10.1007/s00227-019-3538-9 ↩︎
Mumby & Steneck (2008) 10.1016/j.tree.2008.06.011 ↩︎
Alvarez-Filip et al. (2009) 10.1098/rspb.2009.0339 ↩︎ ↩︎
Newman et al. (2015) 10.1111/1365-2656.12429 ↩︎
I don’t know to what extend this applies to other regions. ↩︎
Mumby et al. (2007) 10.1038/nature06252 ↩︎ ↩︎
Mumby (2006) 10.1890/1051-0761(2006)016[0747:TIOEGS]2.0.CO;2 ↩︎ ↩︎
Weiss & Martindale (2017) 10.1371journal.pone.0181637 ↩︎
Bozec et al. (2013) 10.1111/j.1600-0706.2012.20576.x ↩︎
Jompa & McCook (2002) 10.1016/S0022-0981(02)00040-0 ↩︎
Cramer et al. (2017) 10.1038/ncomms14160 ↩︎
Vermeij et al. (2010) 10.1371/journal.pone.0014312 ↩︎